The formula for Density is:
d=m/v
The conversion between Density and Moles is a two step process.
Example:
How many moles are in a 25.0mL sample of Iron if the density of Iron is 7.87g/mL
Step 1: Convert from Density to Mass using the equation.
25.0mL x 7.87g/1mL = 197g
Step 2: Convert from Mass to Moles using Molar Mass
197g x 1mol/55.8g = 3.53mol
Posts by: Aldin Agustin, Christian Arenzana, Kimberley Matibag, & Suban Selvakumaran
Course: Chemistry 11
Teacher: Mr. Doktor
Block: C
Wednesday, 7 December 2011
Saturday, 3 December 2011
Lab 4B from Heath Chemistry Lab Manuel
In class, our the purpose of our Lab was: To find out how many moles, raio of atoms of formula units are produced in the reaction of iron and copper (II) chloride.
Materials: Beaker, Washbottle, Stirring Rod, Tongs, Scapula, Scale, Drying oven, Safety goggles, Filter, Copper (II) Chloride, 2 Iron nails, 50 mL of distilled water
Procedure:
1. Find the mass of a dry 250 mL beaker, the 2 nails, and the piece of paper you are using to place the copper (II) chloride. Record it's mass using the scale.
2. Add the chloride onto the paper until you have reached 7 grams (Make sure it's below 10g)
3. Add the powder into the beaker
4. Weigh the beaker with the Copper (II) Chloride powder and record it's mass.
5. Add 50 mL of distilled water into the beaker filled with the powder. NOTE: To prevent spillage, use a stirring rod.
6. Add the 2 nails into the beaker filled with the solution.
7. Wait 10-15 minutes.
8. Remove the nails from the beaker one by one, with tongs.
9. Holding the nails above the beaker, spray them with water to remove the copper. NOTE: The less water sprayed, the less water to filter.
10. Once the copper is removed from the nails, set them to dry.
11. As the nails are drying, filter the Copper by decanting.
12. Take the filtered paper filled with copper and set it into the drying oven to dry.
13. After both the nails and filter paper of copper are dry, measure their masses.
14. Clean up all lab stations and equipment.
Materials: Beaker, Washbottle, Stirring Rod, Tongs, Scapula, Scale, Drying oven, Safety goggles, Filter, Copper (II) Chloride, 2 Iron nails, 50 mL of distilled water
Procedure:
1. Find the mass of a dry 250 mL beaker, the 2 nails, and the piece of paper you are using to place the copper (II) chloride. Record it's mass using the scale.
2. Add the chloride onto the paper until you have reached 7 grams (Make sure it's below 10g)
3. Add the powder into the beaker
4. Weigh the beaker with the Copper (II) Chloride powder and record it's mass.
5. Add 50 mL of distilled water into the beaker filled with the powder. NOTE: To prevent spillage, use a stirring rod.
6. Add the 2 nails into the beaker filled with the solution.
7. Wait 10-15 minutes.
8. Remove the nails from the beaker one by one, with tongs.
9. Holding the nails above the beaker, spray them with water to remove the copper. NOTE: The less water sprayed, the less water to filter.
10. Once the copper is removed from the nails, set them to dry.
11. As the nails are drying, filter the Copper by decanting.
12. Take the filtered paper filled with copper and set it into the drying oven to dry.
13. After both the nails and filter paper of copper are dry, measure their masses.
14. Clean up all lab stations and equipment.
Tuesday, 29 November 2011
LAB!
In this class, we did a lab to experimentally determine the molar volume of a gas.We had to measure how much mass does butane have and also the molar mass.
The directions for the lab is as follows:
1. Set up the weigh scale.
2. Weigh the mass of the Butane lighter.
3. Fill the sink with water. Make sure to clog it so that water won’t drain.
4. Fill the graduated cylinder with water.
5. Turn it upside down while keeping the water trapped inside.
6. Direct the Lighter under the graduated cylinder.
7. Fill the graduated cylinder with about 20-25mL of Butane.
8. Dry the Butane lighter after use.
9. Weigh the mass of the Butane lighter.
10. Calculate the mass of Butane that was used.
11. Calculate how many moles of Butane are in the mass. ( )
12. Determine the Molar Volume of Butane. ( )
The directions for the lab is as follows:
1. Set up the weigh scale.
2. Weigh the mass of the Butane lighter.
3. Fill the sink with water. Make sure to clog it so that water won’t drain.
4. Fill the graduated cylinder with water.
5. Turn it upside down while keeping the water trapped inside.
6. Direct the Lighter under the graduated cylinder.
7. Fill the graduated cylinder with about 20-25mL of Butane.
8. Dry the Butane lighter after use.
9. Weigh the mass of the Butane lighter.
10. Calculate the mass of Butane that was used.
11. Calculate how many moles of Butane are in the mass. ( )
12. Determine the Molar Volume of Butane. ( )
Multistep Conversions!
Multistep conversions can be used to figure out the mass, moles, volume and even molecules and atoms in a certain chemical equation.
Mass to Volume:
Step 1: Convert mass to moles.
Step 2: Convert moles to volume.
Mass to Molecules:
Step 1: Convert mass to moles.
Step 2: Convert moles to molecules.
Mass to Atoms:
Step 1: Convert mass to moles.
Step 2: Convert moles to molecules.
Step 3: Convert molecules to atoms.
Volume to Mass:
Step 1: Convert volume to moles.
Step 2: Convert moles to mass.
Volume to Molecules:
Step 1: Convert volume to moles.
Step 2: Convert moles to molecules.
Volume to Atoms:
Step 1: Convert volume to moles.
Step 2: Convert moles to molecules.
Step 3: Convert molecules to atoms.
Examples:
1. 5.1g x 1mol/79.9g x (6.02x10^23)/1mol = 3.8 x 10^22
2. 3.62x10^24 x 1mol/(6.02x10^23) x 320g/1mol = 192g
3. 2.94x10^24 x 1mol/(6.02x10^23) x 142g/1mol = 693g
Mass to Volume:
Step 1: Convert mass to moles.
Step 2: Convert moles to volume.
Mass to Molecules:
Step 1: Convert mass to moles.
Step 2: Convert moles to molecules.
Mass to Atoms:
Step 1: Convert mass to moles.
Step 2: Convert moles to molecules.
Step 3: Convert molecules to atoms.
Volume to Mass:
Step 1: Convert volume to moles.
Step 2: Convert moles to mass.
Volume to Molecules:
Step 1: Convert volume to moles.
Step 2: Convert moles to molecules.
Volume to Atoms:
Step 1: Convert volume to moles.
Step 2: Convert moles to molecules.
Step 3: Convert molecules to atoms.
Examples:
1. 5.1g x 1mol/79.9g x (6.02x10^23)/1mol = 3.8 x 10^22
2. 3.62x10^24 x 1mol/(6.02x10^23) x 320g/1mol = 192g
3. 2.94x10^24 x 1mol/(6.02x10^23) x 142g/1mol = 693g
Sunday, 27 November 2011
Converting between Atoms/Molecules & Moles!
It takes one step to convert from Moles to Molecules and two steps from Moles to Atoms.
Moles to Molecules:
The conversion factor for moles to molecules is Avogadro's number (6.02 x 10^2)
Example:
How many water molecules are there in 0.65mol?
Step 1: Lay down conversion equation.
0.65mol x 6.02x10^23molec / 1mol = ???
Step 2: Multiply by Avogadro's number and divide by 1mol (to cancel the mol).
0.65mol x 6.02x10^23molec / 1mol = 3.9x10^23 molec
Moles to Atoms:
Convert Moles to Molecules first by using Avogadro's number as a conversion factor then use subscripts
Example:
How many oxygen atoms are in 3.9x10^23molec?
Step 1: Lay down conversion equation.
3.9x10^23molec x 2atoms/1mol = ???
Step 2: Multiply the molecules by the number of subscripts.
3.9x10^23molec x 2atoms/1mol = 7.8x10^30 oxygen atoms.
Moles to Molecules:
The conversion factor for moles to molecules is Avogadro's number (6.02 x 10^2)
Example:
How many water molecules are there in 0.65mol?
Step 1: Lay down conversion equation.
0.65mol x 6.02x10^23molec / 1mol = ???
Step 2: Multiply by Avogadro's number and divide by 1mol (to cancel the mol).
0.65mol x 6.02x10^23molec / 1mol = 3.9x10^23 molec
Moles to Atoms:
Convert Moles to Molecules first by using Avogadro's number as a conversion factor then use subscripts
Example:
How many oxygen atoms are in 3.9x10^23molec?
Step 1: Lay down conversion equation.
3.9x10^23molec x 2atoms/1mol = ???
Step 2: Multiply the molecules by the number of subscripts.
3.9x10^23molec x 2atoms/1mol = 7.8x10^30 oxygen atoms.
Monday, 21 November 2011
Moles to Volume Conversions
The volume of 1 mol in a substance is molar volume.
At a specific temperature and pressure one mole of any gas occupies at the same volume.
- At 0 °C and 101.3kPa 1 mol = 22.4 L
- This temperature and pressure is called STP
* 22.4 L/mol is the molar volume at STP (Standard Temperature Pressure)
Examples:
At a specific temperature and pressure one mole of any gas occupies at the same volume.
- At 0 °C and 101.3kPa 1 mol = 22.4 L
- This temperature and pressure is called STP
* 22.4 L/mol is the molar volume at STP (Standard Temperature Pressure)
Examples:
Converting from Moles to Mass!
Mr. Doktor explained to us how to convert Moles into Mass.
The mole being converted is multiplied by mass/1 mol.
Ex:
0.89mol x 111.1g/1mol = 98.88g
1.112mol x 20.0g/1mol = 22.2g
0.159mol x 60.1g/1mol = 9.56g
Mr Doktor also showed us how to convert Mass into Moles.
The mass being converted is multiplied by 1 unit of mass/mol.
Ex:
158.1g x 1mol/303.3g = 0.5213mol
362.8g x 1mol/72g = 5.04mol
12.35g x 1mol/58.0g = 0.140mol
The 1mol is either on the top or bottom so that units can cancel.
The mole being converted is multiplied by mass/1 mol.
Ex:
0.89mol x 111.1g/1mol = 98.88g
1.112mol x 20.0g/1mol = 22.2g
0.159mol x 60.1g/1mol = 9.56g
Mr Doktor also showed us how to convert Mass into Moles.
The mass being converted is multiplied by 1 unit of mass/mol.
Ex:
158.1g x 1mol/303.3g = 0.5213mol
362.8g x 1mol/72g = 5.04mol
12.35g x 1mol/58.0g = 0.140mol
The 1mol is either on the top or bottom so that units can cancel.
Thursday, 17 November 2011
Molar Mass!
Today during chemistry class, Mr. Doktor taught us how to find the molar mass of a substance and a compound.
•The mass (in grams) of 1 mole of a substance if called the molar mass.
•It can be determined from the atomic mass on the periodic table.
•It is measure in g/mol
Ex. Iron(Fe) – 55.8 g/mol
Ex. Oxygen (O2) – 2(16) = 32.0 g/mol
Molar Mass of Compounds
• To determine the molar mass of a compound add the mass of all the atoms together.
Ex. NaCl-------- 23 + 35.5 = 58.5 g/mol
Ex. AlCl3------- 27 + 3(35.5) = 133.5 g/mol
•The mass (in grams) of 1 mole of a substance if called the molar mass.
•It can be determined from the atomic mass on the periodic table.
•It is measure in g/mol
Ex. Iron(Fe) – 55.8 g/mol
Ex. Oxygen (O2) – 2(16) = 32.0 g/mol
Molar Mass of Compounds
• To determine the molar mass of a compound add the mass of all the atoms together.
Ex. NaCl-------- 23 + 35.5 = 58.5 g/mol
Ex. AlCl3------- 27 + 3(35.5) = 133.5 g/mol
Wednesday, 9 November 2011
Avagadro's Number (How we count atoms)
Today in chemistry class, Mr Doktor taught us how to calculate how many atoms there in a mole of atoms using the Avagradro's number, we learn that:
-Atoms and molecules are extremely small
-Macroscopic bjects contain too many atoms to count or weigh individually
-Amedeo Avogadro proposed that the number of atoms in 12.00000g of carbon be equal to a constant
-This value is now called Avogadro's number and forms te basis of all quantitative chemistry
-1 mol= 6.02x10^23
-1 pair = 2, 1 dozen = 12, 1 century = 100, 1 mol= 6.02x10^23
e.g. a sample of carbon contains 2.47 x10^25 atoms. How many moles of carbon is this?
-Atoms and molecules are extremely small
-Macroscopic bjects contain too many atoms to count or weigh individually
-Amedeo Avogadro proposed that the number of atoms in 12.00000g of carbon be equal to a constant
-This value is now called Avogadro's number and forms te basis of all quantitative chemistry
-1 mol= 6.02x10^23
-1 pair = 2, 1 dozen = 12, 1 century = 100, 1 mol= 6.02x10^23
e.g. a sample of carbon contains 2.47 x10^25 atoms. How many moles of carbon is this?
Sunday, 6 November 2011
Hydrate Lab
Todays class we did a Lab on Hydrates. We were split into groups of two or three to complete the lab. The purpose of our Lab was to measure how much water was in the hydrate.
We were first taught to use a Bunsen Burner, and how to hold the test tube above the bunsen burner so that we wouldn't hurt ourselves. Before we put any hydrate into our test tube, it is to be measured on to the scale. Next, we would receive some hydrate and put in about 1cm into our test tube, put it on the scale and measure how many grams it is all in all. We then would hold up the test tube above the Bunsen Burner until all the water evaporates, it is then put back onto the scale and measured.
After we have gotten all the measurements needed, we would have to find it's percent error using the formula:
To know you have done the Lab correctly, your percent error should've ranged somewhere below 10%
We were first taught to use a Bunsen Burner, and how to hold the test tube above the bunsen burner so that we wouldn't hurt ourselves. Before we put any hydrate into our test tube, it is to be measured on to the scale. Next, we would receive some hydrate and put in about 1cm into our test tube, put it on the scale and measure how many grams it is all in all. We then would hold up the test tube above the Bunsen Burner until all the water evaporates, it is then put back onto the scale and measured.
After we have gotten all the measurements needed, we would have to find it's percent error using the formula:
To know you have done the Lab correctly, your percent error should've ranged somewhere below 10%
Wednesday, 2 November 2011
Naming Compounds Part 2!
Today Mr Doktor explained to us how to name molecular compounds, acids and bases. There are various rules for naming these compounds:
Molecular Compounds:
- There are 7 Diatomic molecules: H2, N2, O2, F2, Cl2, Br2, I2
- There are 2 Polyatomic molecules: S8, P4
Rules for naming a molecular compound:
- Use the name of the first element
- Second element ends in -ide
- 1st atom usually does not have a prefix (Ex. NO -> Nitrogen monoxide)
- Hydrogen doesn't have a prefix (Ex. H2S -> Hydrogen sulfide)
- Some compounds are to be memorized:
Naming Acids:
- Hydrogen compounds are acids (Ex. HCl -> Hydrochloric acid, H2SO4 -> Sulphuric acid)
- Hydrogen appears first in the formula unless it is part of a polyatomic group (Ex. CH3OOOH -> Acetic Acid)
- Classical rules use the suffix -ic and/or the prefix hydro- (Ex. Sulphuric acid, Hydrochloric acid)
- IUPAC system uses the aqueus hydrogen compound (Ex. HCl (aq) -> Aqueous Hydrogen Chloride)
Naming Bases:
- Cation and OH (Ex. NaOH, Ba(OH)2)
- Use the cation name followed by "hydroxide" (Ex. Sodium Hydroxide, Barium Hydroxide)
Some Acids and Bases:
Molecular Compounds:
- There are 7 Diatomic molecules: H2, N2, O2, F2, Cl2, Br2, I2
- There are 2 Polyatomic molecules: S8, P4
Rules for naming a molecular compound:
- Use the name of the first element
- Second element ends in -ide
- 1st atom usually does not have a prefix (Ex. NO -> Nitrogen monoxide)
- Hydrogen doesn't have a prefix (Ex. H2S -> Hydrogen sulfide)
- Some compounds are to be memorized:
| IUPAC Name | Formula |
|---|---|
| Water | H20 |
| Hydrogen Peroxide | H2O2 |
| Ammonia | NH3 |
| Glucose | C6H12O6 |
| Sucrose | C12H22O11 |
| Methane | CH4 |
| Propane | C3H8 |
| Octane | C8H18 |
| Mathanol | CH3OH |
| Ethanol | C2H5OH |
Naming Acids:
- Hydrogen compounds are acids (Ex. HCl -> Hydrochloric acid, H2SO4 -> Sulphuric acid)
- Hydrogen appears first in the formula unless it is part of a polyatomic group (Ex. CH3OOOH -> Acetic Acid)
- Classical rules use the suffix -ic and/or the prefix hydro- (Ex. Sulphuric acid, Hydrochloric acid)
- IUPAC system uses the aqueus hydrogen compound (Ex. HCl (aq) -> Aqueous Hydrogen Chloride)
Naming Bases:
- Cation and OH (Ex. NaOH, Ba(OH)2)
- Use the cation name followed by "hydroxide" (Ex. Sodium Hydroxide, Barium Hydroxide)
Some Acids and Bases:
| Acid/Base | Compound |
|---|---|
| Hydrochloric Acid | HCl |
| Nitric Acid | HNO3 |
| Suphuric Acid | H2SO4 |
| Phosphoric Acid | H3PO4 |
| Acetic Acid | CH3COOH |
| Ammonia | NH3 |
Friday, 28 October 2011
Naming Compounds!
Today during class, Mr.Doktor taught us how to name compounds and we went over the following:
Chemical Nomenclature
• Today the most common system IUPAC- most chemicals.
- Ions
- Binary Ionic
- Polyatomic Ions
- Hydrates
- Molecular Compounds
- Acids/Bases
Chemical Formulas
• Be aware of the difference between ion and compound formulas.
Examples
Zn^2+…………. ion charge (Zinc Ion)
BaCl2…………..number of ions (Barium Chloride)
Non-Metal Ions
F-………………Fluoride Ion
N^3-……………Nitride Ion
O^2-……………Oxide Ion
AlF3………….Aluminum Fluoride
Na2O…………Sodium Oxide
Fe2S3…………Iron (III) Sulfide
Multivalent Ions
• Some elements can form more than one ion.
- Iron Fe^3+ or Fe^2+
- CopperCu^2+ or Cu^1+
• The top number on the Periodic Table is more common.
• IUPAC uses roman numerals in parenthesis to show the charge.
• Classical(i.e. old) systems uses Latin names of elements and the suffixes –ic (larger charge) and –ous(smaller charge)
Examples
Fe^3+…..Ferric Oxide (Fe2O3)
Fe^2+….Ferrous Oxide (FeO)
Other Classical Names
• Ferr-Iron
• Cupp-Copper
• Mercur-Mercury
• Stann-Tin
• Aunn-Gold
• Plumb-Lead
• Wolf-Tungsten
• Argent-Silver
Old Greek Way
Argentous OxideAg2O
Cuppric Sulfide CuS
IUPAC
CuCl2- Copper (II) Chloride
Cr2O3- Chromium (III) Oxide
Hydrates
• Some compounds can form lattices that bond to water molecules.
• Copper Sulfate
• Sodium Sulfate
• These crystals contain water inside them which can be released by heating,
• To Name Hydrates:
- Write the name of the chemical formula.
- Add a prefix indicating the number of water molecules (mono=1, di=2, tri=3 etc.)
- Add hydrate after the prefix.
Examples
Cu (SO4) •5H2O……….Copper (II) Sulphate Pentahydrate (5 Water)
Li (ClO4) •3H2O………Lithium Hypochlorite Trihydrate (3 Water)
Chemical Nomenclature
• Today the most common system IUPAC- most chemicals.
- Ions
- Binary Ionic
- Polyatomic Ions
- Hydrates
- Molecular Compounds
- Acids/Bases
Chemical Formulas
• Be aware of the difference between ion and compound formulas.
Examples
Zn^2+…………. ion charge (Zinc Ion)
BaCl2…………..number of ions (Barium Chloride)
Non-Metal Ions
F-………………Fluoride Ion
N^3-……………Nitride Ion
O^2-……………Oxide Ion
AlF3………….Aluminum Fluoride
Na2O…………Sodium Oxide
Fe2S3…………Iron (III) Sulfide
Multivalent Ions
• Some elements can form more than one ion.
- Iron Fe^3+ or Fe^2+
- CopperCu^2+ or Cu^1+
• The top number on the Periodic Table is more common.
• IUPAC uses roman numerals in parenthesis to show the charge.
• Classical(i.e. old) systems uses Latin names of elements and the suffixes –ic (larger charge) and –ous(smaller charge)
Examples
Fe^3+…..Ferric Oxide (Fe2O3)
Fe^2+….Ferrous Oxide (FeO)
Other Classical Names
• Ferr-Iron
• Cupp-Copper
• Mercur-Mercury
• Stann-Tin
• Aunn-Gold
• Plumb-Lead
• Wolf-Tungsten
• Argent-Silver
Old Greek Way
Argentous OxideAg2O
Cuppric Sulfide CuS
IUPAC
CuCl2- Copper (II) Chloride
Cr2O3- Chromium (III) Oxide
Hydrates
• Some compounds can form lattices that bond to water molecules.
• Copper Sulfate
• Sodium Sulfate
• These crystals contain water inside them which can be released by heating,
• To Name Hydrates:
- Write the name of the chemical formula.
- Add a prefix indicating the number of water molecules (mono=1, di=2, tri=3 etc.)
- Add hydrate after the prefix.
Examples
Cu (SO4) •5H2O……….Copper (II) Sulphate Pentahydrate (5 Water)
Li (ClO4) •3H2O………Lithium Hypochlorite Trihydrate (3 Water)
Wednesday, 26 October 2011
Electron structure (Electron dot diagrams)
In today class we learned how the electrons are present in each element in either basic element form or as an ion. In an ion the element is either positively or negatively charged and either lose or gain an electron depending on the charge of the element. A negatively charged ion would gain electrons and a positively charged ion would lose electrons.
Drawing Electron Dot diagrams:
• To represent the nucleus, write the atomic symbol
• For individual elements determine the number of valence electrons
• Electrons are represented by dots around the symbol
• Four orbitals (one of each side of the nucleus) each holding a max of 2 electrons
*Each orbital gets 1 electron before they pair up
e.g.
Lewis diagrams for Compounds and Ions:
• In covalent compound, electrons are shared
1. Determine the number of valence electron for each atom in the molecule
2. Place atoms so that valence electrons are shared to fill each orbital
e.g.
Ionic Compounds:
• In ionic compounds electrons transfer from one element to another
• Determine the number of valences on the cation is more than to the anion
• Draw [] around the metal & non metals
• Write the charges outside the brackets
e.g.
Drawing Electron Dot diagrams:
• To represent the nucleus, write the atomic symbol
• For individual elements determine the number of valence electrons
• Electrons are represented by dots around the symbol
• Four orbitals (one of each side of the nucleus) each holding a max of 2 electrons
*Each orbital gets 1 electron before they pair up
e.g.
Lewis diagrams for Compounds and Ions:
• In covalent compound, electrons are shared
1. Determine the number of valence electron for each atom in the molecule
2. Place atoms so that valence electrons are shared to fill each orbital
e.g.
Ionic Compounds:
• In ionic compounds electrons transfer from one element to another
• Determine the number of valences on the cation is more than to the anion
• Draw [] around the metal & non metals
• Write the charges outside the brackets
e.g.
Tuesday, 25 October 2011
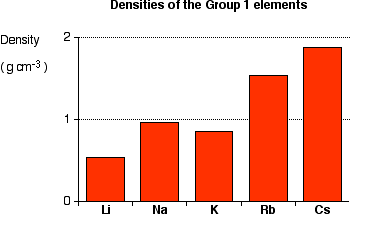
Trends on the Periodic Table
Elements close to eachother on the periodic table display similar characteristics
There are 7 Periodic trends: Reactivity, Ion Charge, Melting Point, Atomic Radius, Ionization energy, Electronegativity, & Density.
Reactivity
Metals and non-metals show different trends.
- The most reactive metal is Francium
- The most reactive non-metal is Fluorine
Ion Charge
Elements ion charges depend on their group/column.
Melting Point
Elements in the center of the table have the highest melting point
- Noble gases have the lowest melting points
- Starting from the left and moving right, melting point increases (until the middle of the table.
Atomic Radius
Radius decrease to the up and the right
- Helium is the smallest atomic radius
- Francium is the largest atomic radius
Ionization Energy
The energy neededx to completely remove an electron from an atom
- it increases going up and to the right
- all Noble Gases have high ionization energy
- Helium (non-metal) has the highest ionoization energy
- Francium (metal) has the lowest ionization energy
*OPPOSITE trend from Atomic Radius
Electronegativity
Refers to how much atoms want to gain electrons
*SAME trend as Ionization Energy
- Francium: wants to gain electrons
Density
How compact an atom is.
-From top to bottom, density increases
There are 7 Periodic trends: Reactivity, Ion Charge, Melting Point, Atomic Radius, Ionization energy, Electronegativity, & Density.
Reactivity
Metals and non-metals show different trends.
- The most reactive metal is Francium
- The most reactive non-metal is Fluorine
Ion Charge
Elements ion charges depend on their group/column.
Melting Point
Elements in the center of the table have the highest melting point
- Noble gases have the lowest melting points
- Starting from the left and moving right, melting point increases (until the middle of the table.
Atomic Radius
Radius decrease to the up and the right
- Helium is the smallest atomic radius
- Francium is the largest atomic radius
Ionization Energy
The energy neededx to completely remove an electron from an atom
- it increases going up and to the right
- all Noble Gases have high ionization energy
- Helium (non-metal) has the highest ionoization energy
- Francium (metal) has the lowest ionization energy
*OPPOSITE trend from Atomic Radius
Electronegativity
Refers to how much atoms want to gain electrons
*SAME trend as Ionization Energy
- Francium: wants to gain electrons
Density
How compact an atom is.
-From top to bottom, density increases
Wednesday, 19 October 2011
Isotopes and Atoms!
Today we learned about atoms and isotopes. No atom is perfectly the same; some atoms have variations of mass (due to the difference in neutron number) and these are called isotopes. We also learned how a spectrometer works and how it gets the average of the different isotopes.
Important Definitions
Atomic Number - Number of protons in an atom
Isotopes - Same atomic number but different mass
Spectrometer - are used to determine the abundance and mass of the isotopes of elements
Isotopes of Hydrogen

Spectrometer: Calculating averages

We have in the image shown above the relative abundances of europium. To find the average mass we need to do a series of calculations...
Step 1: Multiply the percentage (convert to decimals) with the mass number
151(0.478) + 153(0.522) = Average Mass
Step 2: Add the results
72.178 + 79.866 = Average Mass
Step 3: You get your average
152.0 = Average Mass of Europium
*Check the periodic table! Europium has an atomic mass of 152.0!
Important Definitions
Atomic Number - Number of protons in an atom
Isotopes - Same atomic number but different mass
Spectrometer - are used to determine the abundance and mass of the isotopes of elements
Isotopes of Hydrogen

Spectrometer: Calculating averages

We have in the image shown above the relative abundances of europium. To find the average mass we need to do a series of calculations...
Step 1: Multiply the percentage (convert to decimals) with the mass number
151(0.478) + 153(0.522) = Average Mass
Step 2: Add the results
72.178 + 79.866 = Average Mass
Step 3: You get your average
152.0 = Average Mass of Europium
*Check the periodic table! Europium has an atomic mass of 152.0!
Monday, 17 October 2011
Quantum Mechanics
Today in chemistry class we compared the bohr theory and the quantum theory. Mr. Doktor then taught us about electron configuration and the different orbitals. It was confusing but after doing a lot of examples it was clear and we understood what the it was about.
Bohr Theory
- The electron is a particle that must be in orbital in the atom.
Quantum Theory
- The electron is a cloud of negative charge or a wave function.
- Orbitals are areas in 3D space where the electrons most probably are.
- The energy of the electrons is in its vibrational modes- like notes on a guitar string.
- Photons are produced when high energy modes change to lower energy modes.
S Orbitals
- Each orbital holds 2 electrons
P Orbitals
- There are 3 suborbitals
- Each contains 2 electrons
- Total electrons = 6
D Orbitals
- There are 5 suborbitals
- Each contains 2 electrons
- Total electrons = 10
F Orbitals
- There are 7 suborbitals
- Each contains 2 electrons
- Total electrons = 14
Bohr Theory
- The electron is a particle that must be in orbital in the atom.
Quantum Theory
- The electron is a cloud of negative charge or a wave function.
- Orbitals are areas in 3D space where the electrons most probably are.
- The energy of the electrons is in its vibrational modes- like notes on a guitar string.
- Photons are produced when high energy modes change to lower energy modes.
S Orbitals
- Each orbital holds 2 electrons
P Orbitals
- There are 3 suborbitals
- Each contains 2 electrons
- Total electrons = 6
D Orbitals
- There are 5 suborbitals
- Each contains 2 electrons
- Total electrons = 10
F Orbitals
- There are 7 suborbitals
- Each contains 2 electrons
- Total electrons = 14
Thursday, 13 October 2011
Bohr Diagrams
In today's class, it was just a continuation of last class and just went into further study of what elements the main Bohr diagrams are made up of and how to draw them. The drawing of a bohr diagram was just a review from grade 9 so it was pretty easy.
In a bohr diagram:
• PROTONS=ATOMIC NUMBER
• NEUTRONS=ATOMIC MASS
• Atoms are electrically neutral
There are two different models for fluorine that can be used to describe electron configuration:
1. Energy Level Model:
2. Bohr Model:
In a bohr diagram:
• PROTONS=ATOMIC NUMBER
• NEUTRONS=ATOMIC MASS
• Atoms are electrically neutral
There are two different models for fluorine that can be used to describe electron configuration:
1. Energy Level Model:
2. Bohr Model:
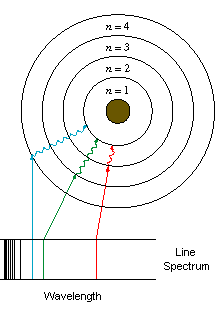
Tuesday, 11 October 2011
Bohr Model
Todays class we had a lesson to learn more about the Bohr Model & deepen our understanding of Niels Bohr's theory.
Bohr's Theory
- Electrons exist in orbitals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light.
BOHR (1920s)
- Rutherfords model was inherently unstable
protons & electrons should attract eachother
- Matter emits light when it is heated
- Light travels as photons and the energy photons carry depends on their wavelength
seperating white light with a diffraction of wavelengths
Each line represents a photon of light emitted from the excited atom
- Bohr based his model on the energy emitted by different atoms
- Each atom has a specific spectrum of light & each spectrum represents an element
Bohr suggested that electrons occupy shells or orbitals to explain this emission.
We also watched a video on YouTube called the Double Slit experiment by Dr Quantam, which shows particles that go through a slit and form a pattern on a backboard, and then figuring out why there is an interference pattern on the back wall when there are 2 slits made to form a pattern on the backboard.
Bohr's Theory
- Electrons exist in orbitals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light.
BOHR (1920s)
- Rutherfords model was inherently unstable
protons & electrons should attract eachother
- Matter emits light when it is heated
- Light travels as photons and the energy photons carry depends on their wavelength
seperating white light with a diffraction of wavelengths
Each line represents a photon of light emitted from the excited atom
- Bohr based his model on the energy emitted by different atoms
- Each atom has a specific spectrum of light & each spectrum represents an element
Bohr suggested that electrons occupy shells or orbitals to explain this emission.
We also watched a video on YouTube called the Double Slit experiment by Dr Quantam, which shows particles that go through a slit and form a pattern on a backboard, and then figuring out why there is an interference pattern on the back wall when there are 2 slits made to form a pattern on the backboard.
Tuesday, 4 October 2011
Atomic Thoeries!
Today we learned about many atomic thoeries throughout history. An atomic thoery relates idea/thoeries about small particles such as atoms, protons, and electrons.
These are some of the atomic thoeries we learned today:
These are some of the atomic thoeries we learned today:
| Atomic Thoery | Main Features | Diagram | Shortcomings/Problems |
| Democritus 300 BC | Talks about the atom as the smallest particle of matter. Defines the atom as an indivisible particle Explains certain natural occurrences such as the existence of elements |
Atom the indivisible particle Atomos (in ancient Greek) means "that which cannot be further broken down into smaller pieces". |
Does not give a scientific view of the atom only a conceptual definition Does not talk about subatomic particles (Electrons, Protons, Neutrons) |
| John Dalton 1800s | Explains a lot of chemical properties such as how atoms combine to form molecules Explains chemical change better than the Particle Theory Confirms the basic Laws of Chemistry: Conservation of Mass & definite Proportions |
The solid sphere model Atoms are seen as solid, indestructible spheres (like billiard balls) |
Does not include the existence of the nucleus Does not explain the existence of ions or isotopes Does not talk about subatomic particles (Electrons, Protons, Neutrons) |
| J.J. Thompson 1850s | Infers on the existence of electrons and protons Introduces the concept of the nucleus Infers on the relative nuclear density and atom mass of different atoms |
The raisin bun Model or the chocolate chip cookie model : Atoms are solid spheres made-up of a solid positive mass (or core) with tiny negative particles embedded in the positive core. |
Does not explain the existence of electrons outside the nucleus does not explain the role of electrons in bonding Does not talk about neutrons therefore can't explain radioactivity and the existence of isotopes |
| Rutherford 1905 | First real modern view of the atom Explains why the electron spins around the nucleus Proposes that the atom is really mostly empty space |
The Planetary Model Famous Gold Leaf Experiment proves that the nucleus is positive and the electrons are outside the nucleus. |
Does not place electrons in definite energy levels around the nucleus Doesn't include neutrons in the nucleus Does Not relate the valence electrons atomic charge |
| Neils Bohr | Explains the role of valence electrons in bonding Relegates the number of valence electrons to the Periods of a periodic table Fully explains ionic and covalent bonding Places electrons in definite energy levels 2 e- in the first 8 e- in the second 8 e- in the third |
Electrons in Definite energy Levels around the nucleus Used atomic spectra to prove that electrons are placed in definite orbitals (called shells) around the nucleus. |
It does not explain the shapes of molecules or other abnormalities that result form unevenly shared pairs of electrons (such as the abnormal behaviour of water, the difference in Carbon-Carbon Bonds between diamond and graphite etc..) |
Monday, 3 October 2011
Density & Graphing!
Today in Chemistry Class, we learnt about Density and Graphing. We learnt that the density of an object is its mass divided by its volume or the equation of:
*Density is usually expressed in Kg/L, Kg/m3, or g/cm3
Graphing (5 important things to remember)
1. Labeled axis
2. Appropriate scale
3. Title
4. Date Points
5. Line of Best Fit
3 things can be done while we work with graphs and that is
* Read the graphs
* Find the slope(rise/run)
* Find the area of the graph
example of a properly constructed graph:
*Density is usually expressed in Kg/L, Kg/m3, or g/cm3
Graphing (5 important things to remember)
1. Labeled axis
2. Appropriate scale
3. Title
4. Date Points
5. Line of Best Fit
3 things can be done while we work with graphs and that is
* Read the graphs
* Find the slope(rise/run)
* Find the area of the graph
example of a properly constructed graph:
Wednesday, 28 September 2011
Dimensional Analysis
Today in Chemistry we went over using Dimensional Analysis. Its basically a fancy word for problem solving and converting units. This was basically a review of math but using formulas we just learned and putting them to work. These formulas made converting much more easier.
Converting is easy as:
1. Identifying what units you to end up with
2. Find the Conversion Factors
3. Place units in the appropriate place
4. Cancel units
e.g.
How many miles are equal to 120 km?
Converting is easy as:
1. Identifying what units you to end up with
2. Find the Conversion Factors
3. Place units in the appropriate place
4. Cancel units
e.g.
How many miles are equal to 120 km?
Sunday, 25 September 2011
Powers of Ten
Todays class Mr. Doktor started by telling the class about some really interesting news about: Scientist breaking the speed of light in Genova, a finding that could overturn one of Einstein's fundamental laws of the universe. Measurements taken over three years showed neutrinos pumped from CERN near Geneva to Gran Sasso in Italy had arrived 60 nanoseconds quicker than light would have done.
We then had a lesson on Significant Digits & Scientific Notation, and Mr. Doktor gave us a worksheet to practice and review.
- All #'s from 1-9 are significant
- When we add/subtract two #'s we determine the number of significant digits by lining up the numbers and then rounding the decimal.
- When we multiply/divide two #'s we determine the # of significant digits by rounding to the #.
Coverting/Expanding
250,000,000 = 2.5x10^8
2.57x10^4 = 257,000
We then had a lesson on Significant Digits & Scientific Notation, and Mr. Doktor gave us a worksheet to practice and review.
- All #'s from 1-9 are significant
- When we add/subtract two #'s we determine the number of significant digits by lining up the numbers and then rounding the decimal.
- When we multiply/divide two #'s we determine the # of significant digits by rounding to the #.
Coverting/Expanding
250,000,000 = 2.5x10^8
2.57x10^4 = 257,000
Thursday, 22 September 2011
SI Units and Error in Physics!
Today Mr. Doktor introduced us SI units and it's prefixes. The SI prefixes indicate how much multiplier (by 10) a unit is measured. For Example: a unit of 1000 can be indicated as 10^3 or in other words "a kilo". These prefixes can be find anywhere from computers to cameras and to any measuring device (Terabyte, kilogram, megapixel, etc.).
These are some of the prefixes in SI Units (and its multipliers):

Error is an unescapable part of science. Measuring instruments are never completely free of flaws and measuring always involves estimation. Three reasons error might ovvur in an experiment could be: The measuring instrument has a flaw, estimates of the human maybe wrong, or chaning ambient conditions of the surroundings may change.
We also learned about Absolute Error and Percent Error. Absolute error and percent error are used to determine how precise and accurate something is.
The formula for Absolute Error is:
Absoulte Error = Measured Value - Accepted Value
The formula for Percent Error is:
Percent Error = [(Measured Value - Accepted Value) / Accepted Value] x 100
These are some of the prefixes in SI Units (and its multipliers):

Error is an unescapable part of science. Measuring instruments are never completely free of flaws and measuring always involves estimation. Three reasons error might ovvur in an experiment could be: The measuring instrument has a flaw, estimates of the human maybe wrong, or chaning ambient conditions of the surroundings may change.
We also learned about Absolute Error and Percent Error. Absolute error and percent error are used to determine how precise and accurate something is.
The formula for Absolute Error is:
Absoulte Error = Measured Value - Accepted Value
The formula for Percent Error is:
Percent Error = [(Measured Value - Accepted Value) / Accepted Value] x 100
Tuesday, 20 September 2011
Homogeneous and Heterogeneous Substances!
In today’s class, Mr. Dockor had briefly gone over a few of the balancing word equations from our homework that’s due next class. After that we had moved on to how we name matter helps us understand it. We learnt that matter can be divided into 2 types, Homogeneous Substances and Heterogeneous Substances. The two types of matter can also be broken further down into smaller groups such as pure substances, elements, compounds, mixtures, solutions and such displayed in the diagram below. Near the end of the class we also saw an example of how a mixture can be separated.
Homogeneous: consists of only one visible component (eg. distilled water, oxygen, graphite)
Heterogeneous: contains more than one visible component (chocolate chip cookie, granite)
2 types of pure substances:
Elements- substances that can’t be broken down into simpler substances by chemical reactions (any element)
Compounds- 2 or more elements that can be changed into other compounds by chemical reactions. (Water, sugar)
Solution- a homogeneous mixture of 2 or more substances (Fog, Steel)
-the component present is greater amounts is the solvent and in smaller amounts is the solute.
Mixtures- heterogeneous mixtures have different parts to it that is clearly visible (blood, sand)
- 5 different ways to separate mixtures
- by hand
- filtration
- distillation
- crystallization
- chromatography
Homogeneous: consists of only one visible component (eg. distilled water, oxygen, graphite)
Heterogeneous: contains more than one visible component (chocolate chip cookie, granite)
2 types of pure substances:
Elements- substances that can’t be broken down into simpler substances by chemical reactions (any element)
Compounds- 2 or more elements that can be changed into other compounds by chemical reactions. (Water, sugar)
Solution- a homogeneous mixture of 2 or more substances (Fog, Steel)
-the component present is greater amounts is the solvent and in smaller amounts is the solute.
Mixtures- heterogeneous mixtures have different parts to it that is clearly visible (blood, sand)
- 5 different ways to separate mixtures
- by hand
- filtration
- distillation
- crystallization
- chromatography
Thursday, 15 September 2011
Physical and Chemical Change:
In today's class we looked over the homework we did last class which was all about balancing. In most cases, the balancing equations part was easy for me but on occasion there were these weird numbers which we had to make into a fraction then do all the math but all in all, it was actually fun for me.
Then we reviewed something we had all learned last year, the changes in matter such as physical and chemical change. One thing i didn't know that was also part of change was nuclear change. Its interest how this all works, you either added energy or removed energy to undergo change.
The three groups are broken down into these:
-Physical Change is just a changing in shape or state of matter
e.g.
-Chemical Change is making new substances or the properties of the matter change
e.g.
-Nuclear Change is a fusion of neutrons and protons or a change of nuclear structure such as fission of a nucleus atom.
e.g.
Then we reviewed something we had all learned last year, the changes in matter such as physical and chemical change. One thing i didn't know that was also part of change was nuclear change. Its interest how this all works, you either added energy or removed energy to undergo change.
The three groups are broken down into these:
-Physical Change is just a changing in shape or state of matter
e.g.
-Chemical Change is making new substances or the properties of the matter change
e.g.
-Nuclear Change is a fusion of neutrons and protons or a change of nuclear structure such as fission of a nucleus atom.
e.g.
Wednesday, 14 September 2011
Balancing Equations!
Todays class we went over our homework, which was a small review of the Lab Safety Rules. Each student then received a green, yellow, and red card to let Mr. Doktor know if we were understanding what he was teaching throughout the lesson. (Green as in I fully understand, Yellow as in I need a few more examples, & Red as in I don't get it at all.)
First we learned what aqueous was (A solution dissolved in liquid) and some other few phase symbols. Ex: Al (s), H2O (l), AgNO3 (aq)
He then listed and explained the Diatomic Molecules: H2, N2, O2, F2, Cl2, Br2, I2
& Polyatomic (many) Molecules: P4, S8
Then Mr. D went through the colummns of the Periodic Table, & explained to us the noble gases, non-metals, and metals. We then took notes on Balancing & Word Equations & were taught an easier way to balance equations without drawing a chart.
An example would be balancing Double Replacement:
2AlCl3 + 3CO2 + 3H2O ---> Al2(CO3)3 + 6HCl
Video taken from kahnacademy.com.
First we learned what aqueous was (A solution dissolved in liquid) and some other few phase symbols. Ex: Al (s), H2O (l), AgNO3 (aq)
He then listed and explained the Diatomic Molecules: H2, N2, O2, F2, Cl2, Br2, I2
& Polyatomic (many) Molecules: P4, S8
Then Mr. D went through the colummns of the Periodic Table, & explained to us the noble gases, non-metals, and metals. We then took notes on Balancing & Word Equations & were taught an easier way to balance equations without drawing a chart.
An example would be balancing Double Replacement:
2AlCl3 + 3CO2 + 3H2O ---> Al2(CO3)3 + 6HCl
Video taken from kahnacademy.com.
Saturday, 10 September 2011
Lab Prep! Rules and Safety in the Laboratory 101
Today for class, Mr Doktor explained to us various Safety procedures when doing an experiment in the laboratory. He made us get into groups and create our own "Top 10 Safety Rules" to help create an official safety list for the class. During the class he also showed us a video which enlightened us how not applying safety precautions during experiments can lead to dangerous or even deadly consequences. So overall, in today's class we learned that we should learn the proper safety precautions in a lab to ensure our safety and others as well.
An example of a Top 10 Science Safety that was made:
1. Wear lab coats and safety goggles
2. No horseplay (running)
3. No touching, tasting, directly smelling chemicals
4. Listen to everything Mr. Doktor says
6. No eating during lab experiments
7. Raad labels before using chemicals
8. Don't leave an experiment unattended (especially Bunsen Burners)
9. Use the appropriate procedures in an emergency
10. Alert Mr. Doktor if anyone is hurt or if something breaks
An example of a Top 10 Science Safety that was made:
1. Wear lab coats and safety goggles
2. No horseplay (running)
3. No touching, tasting, directly smelling chemicals
4. Listen to everything Mr. Doktor says
6. No eating during lab experiments
7. Raad labels before using chemicals
8. Don't leave an experiment unattended (especially Bunsen Burners)
9. Use the appropriate procedures in an emergency
10. Alert Mr. Doktor if anyone is hurt or if something breaks
Wednesday, 7 September 2011
Welcome to our Chemistry 11 Blog 2011-2012!
Bloggers: Aldin Agustin, Christian Arenzana, Kimberley Matibag, Suban Selvakumaran
Course: Chemistry 11
Teacher: Mr. Doktor
Block: C
CHEMISTRY 11 LEGGO!
Course: Chemistry 11
Teacher: Mr. Doktor
Block: C
CHEMISTRY 11 LEGGO!
Subscribe to:
Comments (Atom)