Todays class we had a lesson to learn more about the Bohr Model & deepen our understanding of Niels Bohr's theory.
Bohr's Theory
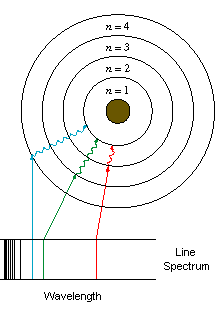
- Electrons exist in orbitals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light.
BOHR (1920s)
- Rutherfords model was inherently unstable
protons & electrons should attract eachother
- Matter emits light when it is heated
- Light travels as photons and the energy photons carry depends on their wavelength
seperating white light with a diffraction of wavelengths
Each line represents a photon of light emitted from the excited atom
- Bohr based his model on the energy emitted by different atoms
- Each atom has a specific spectrum of light & each spectrum represents an element
Bohr suggested that electrons occupy shells or orbitals to explain this emission.
We also watched a video on YouTube called the Double Slit experiment by Dr Quantam, which shows particles that go through a slit and form a pattern on a backboard, and then figuring out why there is an interference pattern on the back wall when there are 2 slits made to form a pattern on the backboard.

No comments:
Post a Comment