Elements close to eachother on the periodic table display similar characteristics
There are 7 Periodic trends: Reactivity, Ion Charge, Melting Point, Atomic Radius, Ionization energy, Electronegativity, & Density.
Reactivity
Metals and non-metals show different trends.
- The most reactive metal is Francium
- The most reactive non-metal is Fluorine
Ion Charge
Elements ion charges depend on their group/column.
Melting Point
Elements in the center of the table have the highest melting point
- Noble gases have the lowest melting points
- Starting from the left and moving right, melting point increases (until the middle of the table.
Atomic Radius
Radius decrease to the up and the right
- Helium is the smallest atomic radius
- Francium is the largest atomic radius
Ionization Energy
The energy neededx to completely remove an electron from an atom
- it increases going up and to the right
- all Noble Gases have high ionization energy
- Helium (non-metal) has the highest ionoization energy
- Francium (metal) has the lowest ionization energy
*OPPOSITE trend from Atomic Radius
Electronegativity
Refers to how much atoms want to gain electrons
*SAME trend as Ionization Energy
- Francium: wants to gain electrons
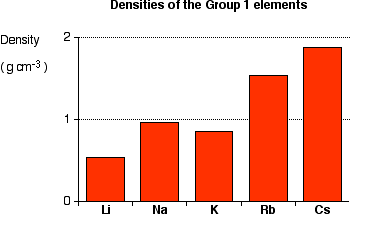
Density
How compact an atom is.
-From top to bottom, density increases


No comments:
Post a Comment