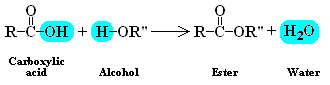
Nomenclature:
- Write down the side chain without the double bonded oxygen
- Have the side chain with the double bonded oxygen end in -oate
- Standard naming rules apply.
Examples:
- Esters also have distictive fruit-like odours!
Heres a video that futher explains Esters:




